Tuesday, November 5, 2002
1111
Multicenter Evaluation of an External Tissue Expander System (Brava®) for Breast Enlargement
Purpose: Tissue expansion is a proven technology that generates tissue. We conducted a multicenter study to confirm the feasibility of breast enlargement with the Brava external breast expansion system and to determine the factors conducive to good results.
Method: After obtaining IRB approval, 21 surgeons from four regions of the US referred 125 women (age18-51 av. 34) to 6 clinical research centers. Upon enrolment, at the end of the study, and at least six times in the interim, questionnaires, breast examinations, standardized photographs and volume measurements by bead displacement were obtained. The women were not compensated and received the bra-like external tissue expansion system free of charge. They were asked to wear it for >10 hours/day for at least 10 consecutive weeks. Final recordings were obtained at least one month after cessation of treatment and only after unchanged breast volume measurements two weeks apart confirmed stable enlargement and completion of the recoil. Results were correlated with demographic and physical factors, with duration of treatment and with compliance as recorded by the microchip in the device.
Results: 95 women completed 10-20weeks of treatment (average 13w). After 2months reprieve, 8 women underwent another treatment cycle. Reasons for drop out were: non-compliance with the lengthy commitment (24); refractory dermatitis (3); and >5% body weight change (3).
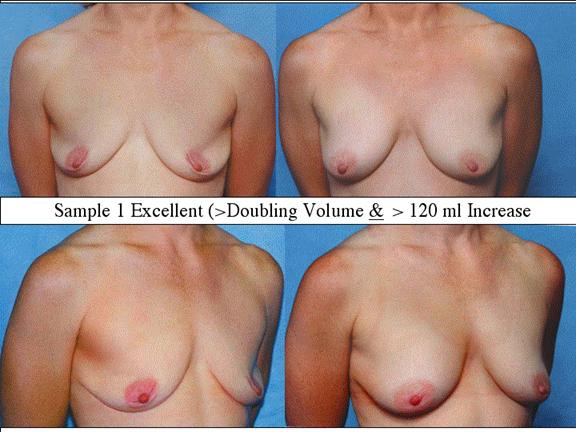
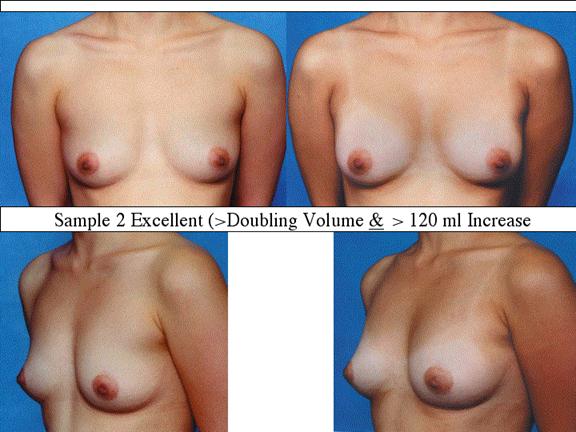
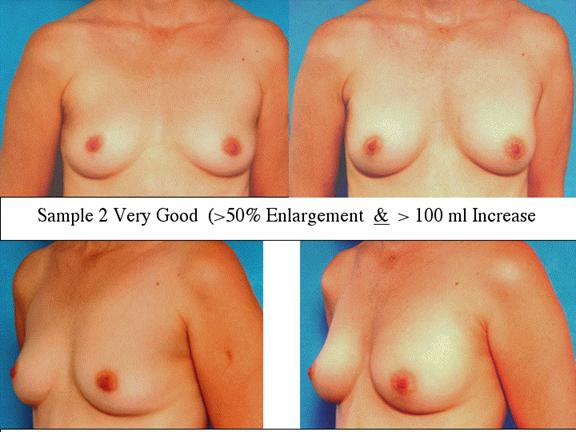
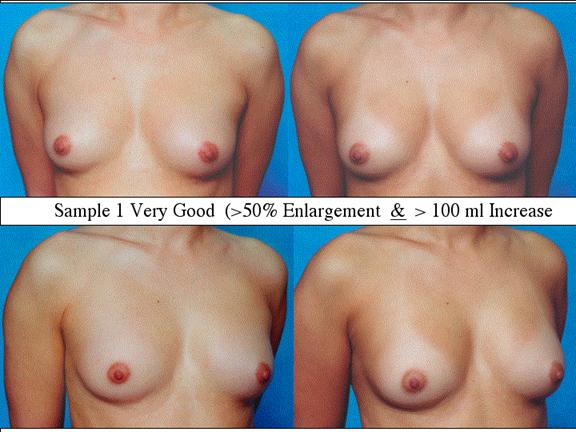
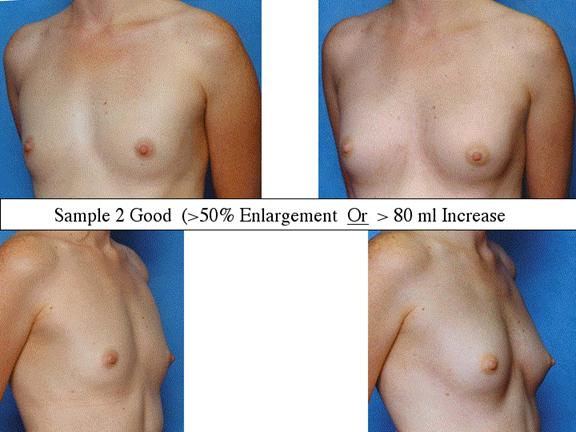
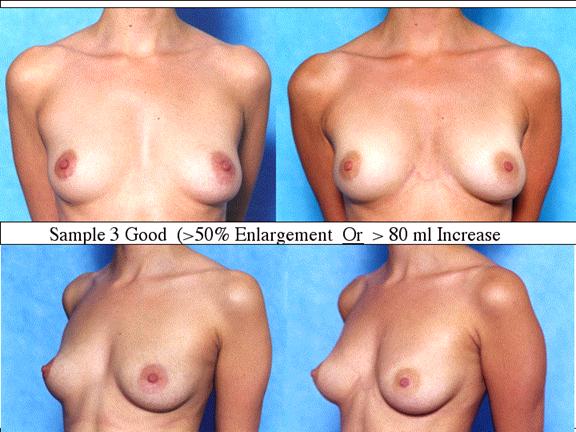
At final follow-up, stable breast enlargement achieved was 108±38ml (range 30–250ml, median 105ml). This corresponds to an average 80% increase in breast volume (range 11–290%). The results were categorized as excellent (n=14) if they more than doubled their original breast volume and achieved >120ml volume increase; very good (n=43) if > 50% and >100ml; good (n=27) if >50% or >80ml; and marginal (n=11) if <50% and <80ml.
Parity, race, original breast volume and body fat index had some effect that was largely overshadowed by the effect of compliance (ratio of on/off expansion time) and duration of treatment. Resultant breast enlargement strongly correlated with compliance (%time used for >10 hours/day) and with the length of the overall treatment period.
Conclusions: This multicenter study confirms that external tissue expansion of the breast leads to larger fuller breasts. Like other tissue expansion procedures true tissue growth is slow and requires sustained tension. The stiff compliance requirement of this external tissue expander and the slow tissue growth rate of approximately 1 to 1.5 ml/day compound the issue. Brava offers compliant patients with patience a non-surgical alternative for moderate breast enlargement.