Sunday, October 26, 2003
3424
Reduction of Adhesions Using a Composite Alloderm/Polypropylene Mesh for Abdominal Wall Reconstruction
Introduction: Abdominal wall reconstruction commonly includes the use of structural prosthetic materials such as polypropylene (PP) mesh. PP mesh placed adjacent to peritoneal structures, however, causes dense abdominal adhesions, makes re-operation more complicated, and increases the risk of bowel obstruction and fistula. We previously demonstrated that adhesions to PP mesh can be significantly reduced by using composite mesh constructed with an acellular synthetic dermal analogue (collagen-glycosaminoglycan matrix) incorporated into PP mesh. Alloderm, a commercially available, allogeneic, decellularized human dermal analogue, consists of a preformed, three-dimensional collagen matrix with native basement membrane (BM) components on one surface; this material is gradually revascularized and replaced with autologous tissue. We hypothesized that Alloderm can be integrated with PP mesh to reduce adhesions and serve as a biodegradable scaffold to generate an autologous vascularized tissue layer separating and protecting the abdominal viscera from the PP mesh.
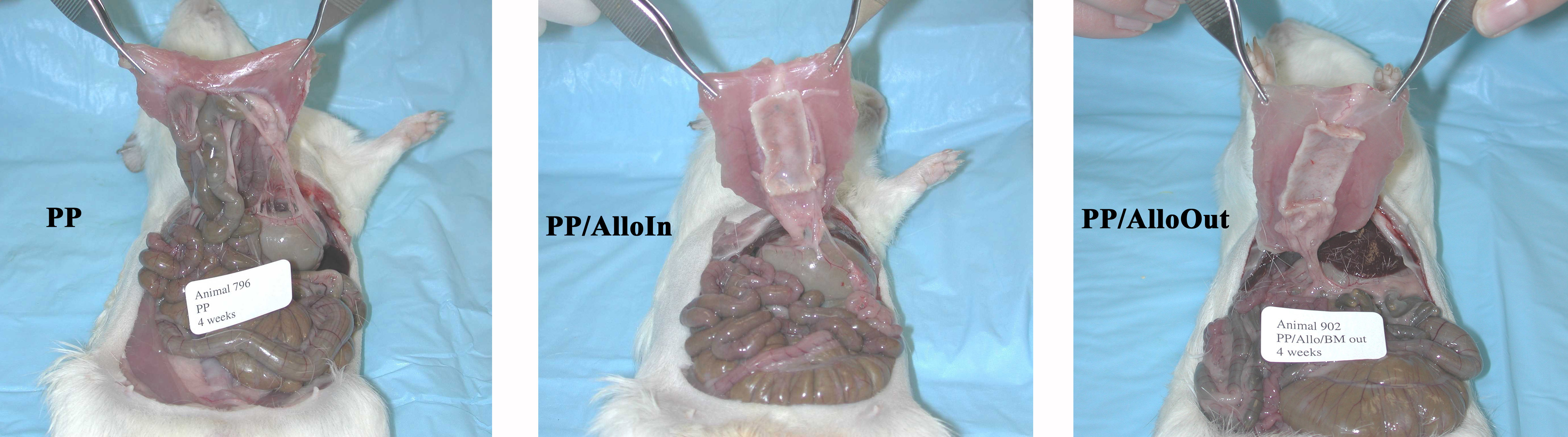
Materials and Methods: Ventral hernia defects (3 x 1 cm) in 19 guinea pigs were repaired with composite mesh implants or PP mesh alone (PP, n=6) using an inlay technique. Composite mesh implants were constructed by integrating PP mesh and Alloderm with its BM surface oriented toward (PP/AlloIn, n=7) or away from (PP/AlloOut, n=6) the peritoneal cavity. Repair sites were widely exposed and analyzed at 4 weeks. The mesh implant surface area involved by adhesion, strength of adhesions (graded from 0 to 3), and incidence of bowel adhesions were determined. Histologic, histomorphologic, and immunohistologic analyses were performed on full-thickness tissue sections from the repair sites.
Results: The mean surface area involved by adhesions and mean adhesion strength were significantly lower in the PP/AlloIn (area, 12.4%; mean grade, 1.0) and PP/AlloOut (9.5%; 0.5) groups than in the PP group (79.5%; 2.9). There were no differences in the surface area involved or strength of adhesions between the PP/AlloIn and PP/AlloOut groups. Bowel was adherent to 72% of PP repairs and 0% of the composite mesh repairs. (See Figures below)
The Alloderm was remodeled to form a vascularized tissue layer beneath the mesh with a mean thickness of 634 and 541 µm in the PP/AlloIn and PP/AlloOut groups, respectively, unlike the significantly thinner dense scar layer (52 µm thickness) that formed in the PP group. A separate, distinct (sub-Alloderm) inflammatory infiltrate was observed beneath the remodeled Alloderm layer, adjacent to the peritoneal cavity in composite but not PP repairs. There were no significant differences in the degree of cellular infiltration or thickness of the vascularized Alloderm layer, or the thickness or composition of the sub-Alloderm inflammatory infiltrate between the PP/AlloIn and PP/AlloOut groups. Immunohistochemical labeling for type IV collagen and laminin localized the BM zone in the incompletely degraded Alloderm. Factor VIII labeling showed neovascularization throughout the Alloderm and focally enhanced vascularity along the BM zone, regardless of BM surface orientation.
Discussion: The Alloderm functioned as a biodegradable tissue scaffold guiding the formation of a thick, well-vascularized tissue layer separating the PP mesh from intraperitoneal structures. This significantly reduced both the surface area involved by adhesions and adhesion strength. BM orientation did not significantly affect adhesion formation, cellular infiltration into or thickness of the remodeled Alloderm layer or sub-Alloderm inflammatory cell response. However, the BM components were associated with enhanced focal neovascularization.
Conclusions: Composite mesh implants composed of structural prosthetic materials integrated with Alloderm may have useful clinical applications for abdominal wall reconstruction by reducing adhesions and providing a vascularized tissue layer to separate and protect the peritoneal structures from PP mesh fibers. This may reduce the morbidity associated with prosthetic mesh reconstruction and facilitate subsequent re-operative abdominal surgery, if required. The BM orientation of the Alloderm layer does not appear to affect the anti-adhesive properties of this composite mesh.
View Synopsis (.doc format, 467.0 kb)
See more of General Reconstruction
Back to Plastic Surgery 2003 Complete Scientific Program
Back to Plastic Surgery 2003 Meeting home
