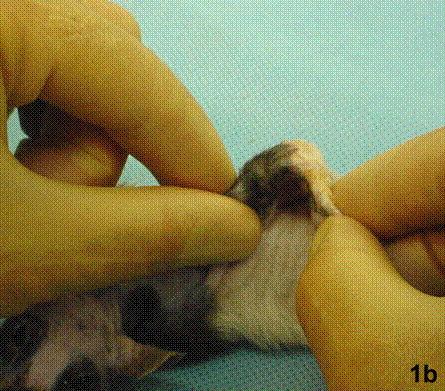Sunday, October 26, 2003
3426
Tissue-Engineered Flexible Ear-Shaped Cartilage
Introduction
Total reconstruction of the external ear currently requires a subcutaneously placed ear-shaped framework of either alloplastic material or autologous costal cartilage to recreate the complex three-dimensional shape of the auricle. Tissue engineering of cartilaginous ear-shaped frameworks has also been attempted; however, the resultant cartilage is small, brittle and inflexible. We previously demonstrated the importance of perichondrium in maintaining the flexibility of elastic cartilage (Xu et al, 2001). This study investigates the use of lyophilized perichondrium as a pseudoperichondrium for engineering flexible, ear-shaped cartilage in rats.
Methods
Sterile technique was used to harvest auricular cartilage and perichondrium from 3 to 6 month old Yorkshire swine. Chondrocytes were isolated using collagenase digestion, suspended in fibrin glue at a concentration of 40 million cells/ml, and polymerized in an ear-shaped mold. Following lyophilization, perichondrium was then added to both sides of the chondrocyte/fibrin glue suspension to form a tri-layer construct which was implanted into a dorsal, subcutaneous pocket of a nude rat. Construct shape was maintained with external stenting for 6 weeks. At 12 weeks both the engineered construct and native swine ear tissue were analyzed with gross mechanical testing and histology.
Results
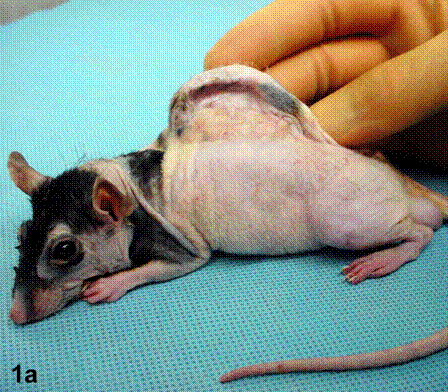
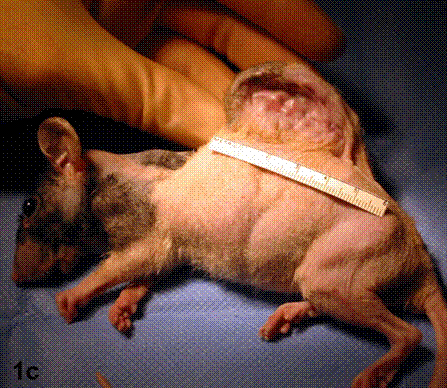
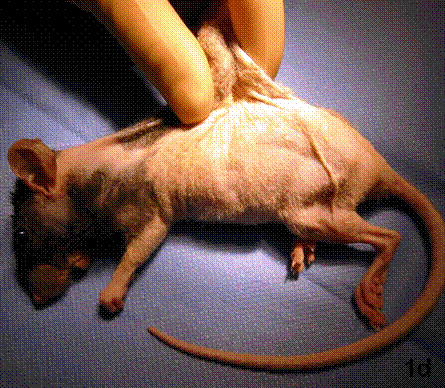
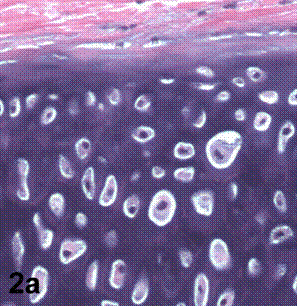
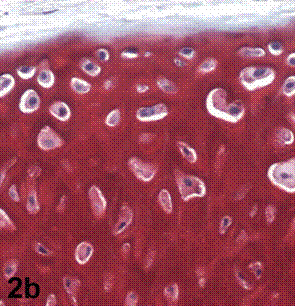
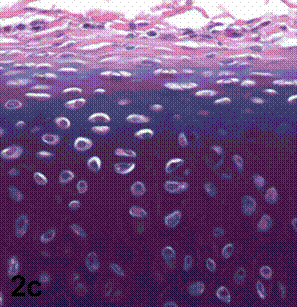
After removal of the stent at 6 weeks a flexible, ear-shaped construct was present (Fig.1a, b). The engineered ear could not be fractured with gross mechanical testing and flexibility was similar to that of the native swine ear. Size, shape and flexibility of the engineered ear were stable at 12 weeks (Figs.1c, d). Histology of the construct revealed a mature neocartilage framework with typical appearing lacunae closely integrated with the lyophilized perichondrium (Fig.2a). Safranin-O staining revealed abundant evidence of sulfated glycosaminoglycans indicating active production of new cartilage matrix (Fig.2b). Cellular architecture of the experimental samples at 12 weeks was similar in appearance to native swine ear tissue (Fig.2c).
Conclusions
This study demonstrates that it is possible to engineer flexible, ear-shaped cartilage using lyophilized perichondrium as a pseudoperichondrium. Furthermore, the cellular architecture is nearly identical to that of native swine auricular cartilage. Simulation of the multiple layers characteristic of elastic cartilage appears to be crucial when engineering flexible cartilage for reconstructive purposes and may result in a new technique for total reconstruction of the external ear.