Wednesday, October 29, 2003
3957
Brow Fixation with the EndotineTM Device in Endoscopic Browplasty
|
Purpose: Numerous brow fixation techniques have evolved over the last ten years with the advent of endoscopic browplasty. Most of these techniques rely upon a single loop of suture material (per side) fixed to the cranium (implantable screws, bone tunnels, etc.), removable percutaneous devices (pins, screws, or wires), or external sutures or dressings. These methods have a number of shortcomings including single point fixation and a lack of reliability. The Endotine™ (Coapt Systems, Palo Alto, CA) is an implantable bioabsorbable fixation device designed to provide intuitive, multi-point distributed tension and predictable brow fixation during endoscopic browplasty. The purpose of this study was to evaluate early results in a series of endoscopic browplasty cases performed with the Endotine™ device.
Materials and Methods: The Endotine™ device used in this study is comprised of polylactic acid. The device consists of a post that is inserted into the cranium (4.25 mm), a platform (1.0 mm), and a series of five tines projecting cephalad from this platform (3.5 mm). These tines serve to puncture the periosteum and galea, thus securing the elevated brow. On a consecutive series of endoscopic browplasty cases, pre- and post-operative standardized photographs in Frankfort horizontal were obtained and three measurements were compared (mid-pupil to superior brow, mid-pupil to hairline, and lateral canthus to superior brow).
Results: A total of 14 patients (thirteen female and one male) underwent endoscopic browplasty. Pre-operative photographs were obtained within one month of the procedure, and follow-up photographs were taken at 54 to 174 days (avg. 109) post-operatively. The following average brow elevation measurements (mm) were obtained:
|
|||
|
|
Pre-operative |
Post-operative |
Elevation ± SD |
|
Mid-pupil to superior brow |
22.4 |
26.7 |
4.52 ± 2.66 |
|
Mid-pupil to hairline |
76.5 |
81.4 |
4.63 ± 2.12 |
|
Lateral canthus to sup. brow |
23.5 |
27.5 |
4.23 ± 2.4 |
No significant adverse events were encountered in the follow-up period. A sample pre- and post-operative image set is shown in Figures 1 and 2.
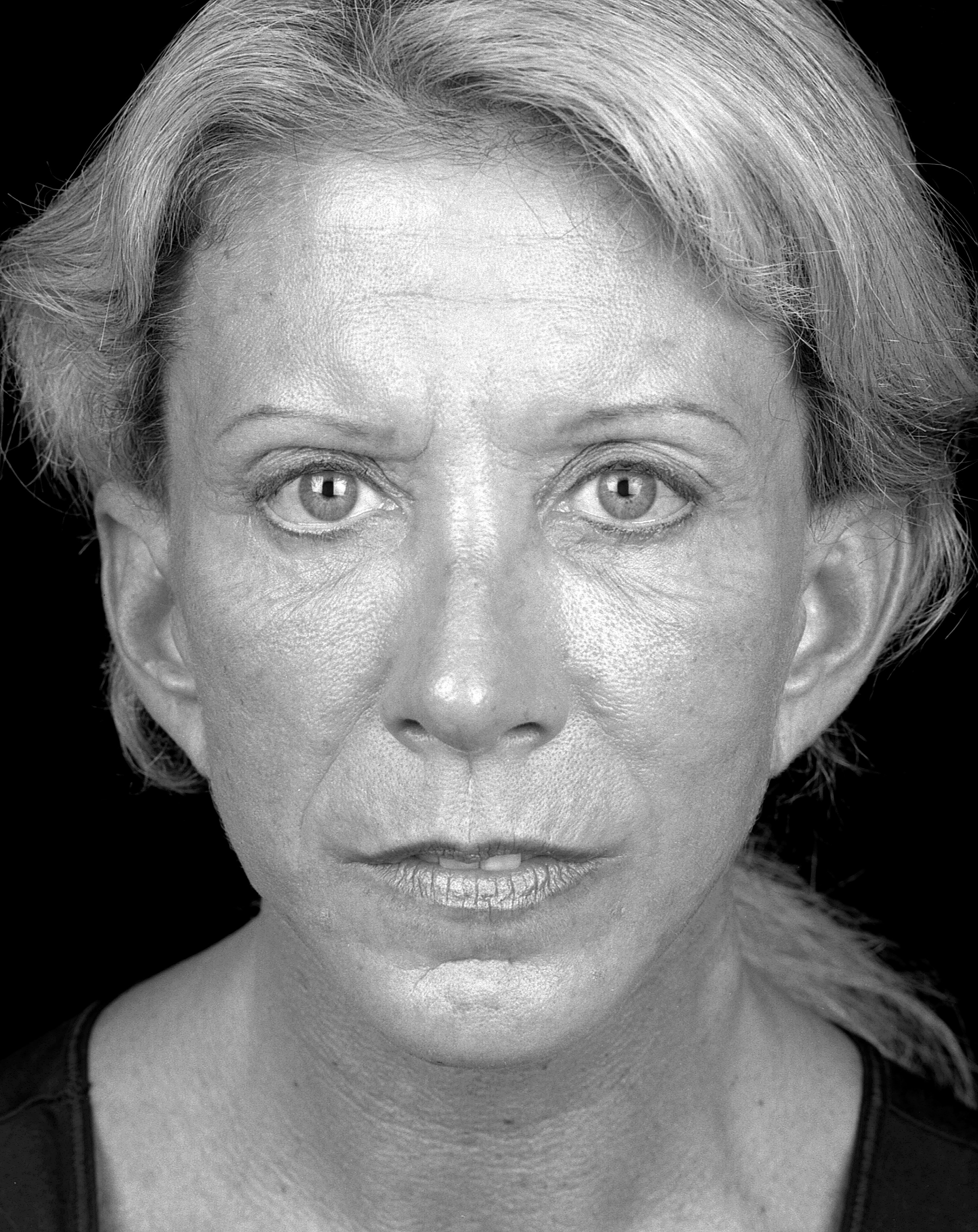
Figure 1: Pre-operative.
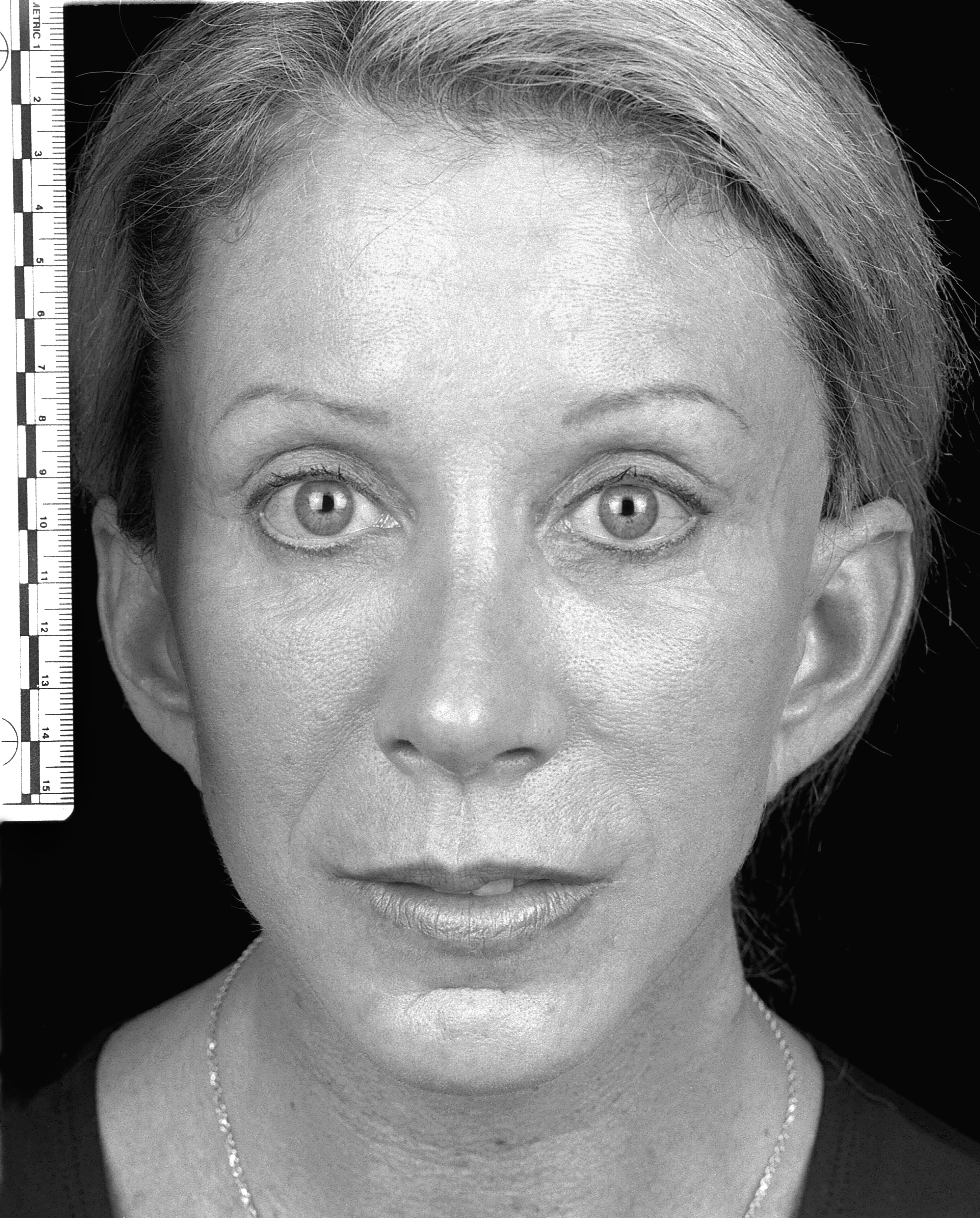
Figure 2: Post-operative (60 days).
Conclusions: The Endotine™ device provides significant and reproducible brow elevation with no significant adverse events as measured at three points in excess of fifteen weeks post-operatively.
