McCormick Place, Lakeside Center
Sunday, September 25, 2005
9:00 AM - 5:00 PM
McCormick Place, Lakeside Center
Monday, September 26, 2005
9:00 AM - 5:00 PM
McCormick Place, Lakeside Center
Tuesday, September 27, 2005
9:00 AM - 5:00 PM
McCormick Place, Lakeside Center
Wednesday, September 28, 2005
9:00 AM - 5:00 PM
8054
Effectiveness of Cryo-Preserved Human Bone Marrow Stromal Cells at Forming Bone in Vivo
Purpose
Cultured bone marrow stromal cells (BMSCs) contain osteoprogenitor cells which provide the cellular basis for bone-directed tissue generation. The majority of BMSC studies involve the transplantation of cells which have undergone recent harvest and culture expansion. Any anticipated clinical trial may require that some cells undergo a period of cryo-preservation, in order to match cell availability with clinical need. In this study, we compared bone formation among freshly-expanded cells with those cells which have been cryo-preserved.
In brief, human bone marrow isolates underwent a low number of culture passages and were then divided into 2 groups, one of which was immediately transplanted into mice, and the other of which was cryo-preserved for an extended period of time, thawed, re-expanded, and then transplanted into a second set of mice. Bone from the 2 groups of transplantations was compared.
Methods
Multi-colony derived strains of BMSCs were obtained from patient bone marrow as previously described.(Kuznetsov, etal; JBMR; 1997) The cells were isolated in tissue culture and expanded via sequential passaging. Aliquots of cells from passage 2 and 3 (for Hum-40 cells) and from passage 1 (for Hum-65 cells) were cryo-preserved in a freezing solution consisting of aMEM, 50% fetal bovine serum of a pre-selected lot, 100 U/mL penicillin, and 5% DMSO (Sigma: St. Louis, Missouri). Cells which had never been frozen, from passages 2 through 4, were combined with hydroxyapatite/tricalcium phosphate (HA/TCP) particles and transplanted into three month old immunodeficient Bg-Nu-Xid female mice using our standard technique.(Mankani, etal; Biotechnol Bioeng; 2001)
Cryo-preserved cells were recovered after freezing periods of 15 weeks (Hum-40) and 13 to 37 weeks (Hum-65), reconstituted in our standard culture media, passaged 1 to 3 times more, combined with HA/TCP particles, and then transplanted into a second set of three month old immunodeficient Bg-Nu-Xid female mice.
All transplants were recovered from periods of 8 weeks to 67 weeks. Transplants arising from freshly utilized BMCSs were harvested at an average of 48 weeks, while transplants from the cryo-preserved progeny of these cells were harvested at an average of 23 weeks. HE-stained sections of the transplants were then examined histologically; the extent of bone within each transplant was scored on a semiquantitative, logarithmic scale by 3 independent, blinded observers:
Semiquantitative scale for the estimation of bone formation
| Score | Extent of bone present within the transplant |
| 0 | No bone evident |
| 1 | Minimal bone evident (1 trabecula) |
| 2 | Weak bone formation, occupying only a small portion of the section |
| 3 | Moderate bone formation, occupying a significant portion but less than one half of the section |
| 4 | Abundant bone formation, occupying greater than one half of the section |
Results
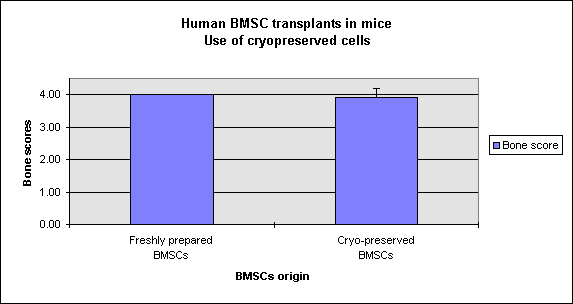
The freshly-prepared and cryo-preserved BMSCs were uniformly successful at forming extensive bone (Figure 1), and the mean bone scores of each group (4.00 vs. 3.92, respectively) were statistically equivalent.
Conclusion
The clinical use of cultured osteoprogenitor cells may require an interval of cryo-preservation prior to transplantation. In this study, freshly-expanded BMSCs and cryo-preserved BMSCs originating from the freshly-expanded BMSCs formed bone which was equivalent, suggesting that cryo-preservation did not impair bone formation.
Figure 1