Sunday, September 25, 2005 - 11:30 AM
8197
Avoidance and Treatment of Malar Implant Deformities
PURPOSE: 1) To document reasons for patient dissatisfaction after malar implant surgery. 2) To present techniques to avoid iatrogenic problems during primary surgery and correct iatrogenic deformities during revisional surgery.
METHODS: A retrospective review of 18 cases presenting for secondary surgery and 35 patients presenting for primary surgery.
RESULTS: There were three main reasons for patient dissatisfaction: 1. implant asymmetry 2. displeasing contours – too wide, too large, too low or too prominent with time 3. infraorbital nerve compression
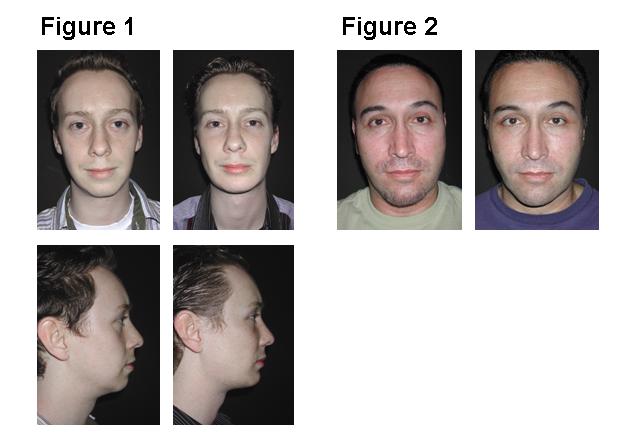
Successful primary surgery depends on the following concepts and techniques: 1) Avoid large, non-anatomically shaped implants. 2) Avoid augmenting the zygomatic arch. 3) Do not use implants to correct soft tissue deficiencies or surface irregularities. 4) Wide subperiosteal exposure through an intraoral approach allows identification of the area to be augmented , landmarks for orientation, and the infraorbital nerve. 5) Porous implants limit the soft tissue contracture deformity. 6) Screw fixation of the implant: prevents movement, obliterates gaps between the implant and the skeleton, allows in- place, precise contouring. Revisional Surgery requires the following steps: 1) Remove the displeasing implants 2) Replace with appropriately positioned and sized implants.3) Resuspend the cheek soft tissue envelope (midface lift) to mask and redistribute the soft tissue distortions. Using these concepts and techniques, one patient in this series of primary and secondary patients required revisional surgery. Patients who underwent primary (Figure 1) and secondary (Figure 2) surgery are presented below.
CONCLUSIONS: – Malar implant deformities are avoided by wide exposure of the skeleton, precise augmentation of the deficient area to create a normal anatomy, and screw fixation of the implant. Malar implants should not be used to expand the soft tissue envelope.
View Synopsis (.doc format, 136.0 kb)