McCormick Place, Lakeside Center
Sunday, September 25, 2005
9:00 AM - 5:00 PM
McCormick Place, Lakeside Center
Monday, September 26, 2005
9:00 AM - 5:00 PM
McCormick Place, Lakeside Center
Tuesday, September 27, 2005
9:00 AM - 5:00 PM
McCormick Place, Lakeside Center
Wednesday, September 28, 2005
9:00 AM - 5:00 PM
8372
Artecoll Injection for Improved Projection in Nipple Reconstruction: a Clinical Trial
INTRODUCTION: Various techniques have been used in an attempt to achieve long-term nipple projection for breast reconstruction patients. Irrespective of the technique used, a common disappointment is loss of projection over time. This phenomenon is particularly evident following implant-based breast reconstruction.
As a means to increase projection after nipple reconstruction, Artecoll injection may be useful. Artecoll is an injectable substance that consists of inert, non-biodegradable polymethylmethacrylate (PMMA) microspheres suspended in a partially denatured 3.5% bovine collagen. The purpose of this study was to prospectively evaluate the efficacy of Artecoll in augmenting and maintaining nipple projection following reconstruction.
PATIENTS & METHODS: A prospective, clinical trial was performed. Seventeen nipples (12 patients) were deemed to have inadequate nipple projection following ‘C-V flap' or ‘modified-skate flap' nipple reconstruction. Nine patients had prior tissue expander/implant breast reconstruction; three patients had prior TRAM flap reconstruction. All 12 patients underwent Artecoll injection at least six months after completion of nipple reconstruction. Skin testing for collagen sensitivity was performed in all patients one month prior to study initiation.
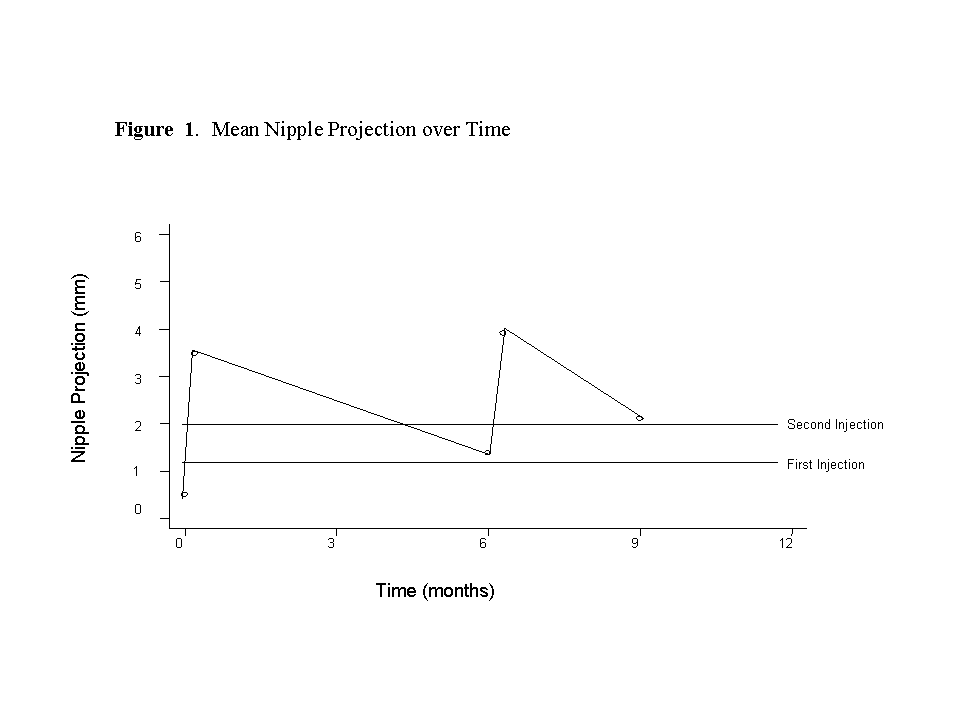
0.6 cc of Artecoll was injected under the nipple at two time points: baseline and 6 months. Calipers were used to measure nipple projection at 5 time points: pre-and post-first injection, pre-and post-second injection at 6 months and at again at 9 months (no injection performed).Descriptive statistics were performed. Pairwise differences in nipple projection were calculated using the Wilcoxon signed rank test. Statistical significance was evaluated at the p=0.05 level.
RESULTS: In total, 17 nipples were injected (12 patients). There were no adverse events related to the Artecoll injections. Complete 9-month follow-up data was available for 14 nipples (10 patients). Prior to injection, mean nipple projection was 0.50 ± 033 mm (range 0.0–1.0 mm). Mean nipple projection immediately following a single injection of Artecoll was 3.48 ± 1.07 mm (range 2.0–5.0 mm). At 6 months post-injection, mean nipple projection was 3.9± 1.2 mm (range 2.5-5.5 mm). At 9 months, mean nipple projection was 2.11± 0.93 mm (range 0.8-4.0 mm). When stratified by method of reconstruction, mean final projection in implant-based reconstruction patients was 2.31 ± 0.93 (range 0.8-4.0 mm; n=12) and mean projection in TRAM flap patients was 1.0 ± 0.0 mm (n=2). The mean increase in nipple projection over the 9 month follow-up period was 1.61 mm (range 0.8 – 3.5 mm; n=14). This increase in projection was both clinically and statistically significant (p=0.002). (Figure 1)
CONCLUSIONS: The subcutaneous injection of Artecoll resulted in a significant and sustained increase in nipple projection. This technique appears most useful in implant-based reconstructions, likely due to the fact that the implant provides a stable base and increases the prominence of the subcutaneous injection. Artecoll injection is both feasible and effective in increasing nipple projection in the setting of implant-based breast reconstruction.
View Synopsis (.doc format, 51.0 kb)