McCormick Place, Lakeside Center
Sunday, September 25, 2005
9:00 AM - 5:00 PM
McCormick Place, Lakeside Center
Monday, September 26, 2005
9:00 AM - 5:00 PM
McCormick Place, Lakeside Center
Tuesday, September 27, 2005
9:00 AM - 5:00 PM
McCormick Place, Lakeside Center
Wednesday, September 28, 2005
9:00 AM - 5:00 PM
8747
“Mapping the Gap”: Combined Histologic and µCT Assessment of Bone Healing During Mandibular Distraction Osteogenesis
Introduction: Despite the increasing clinical use of distraction osteogenesis (DO) of the mandible, the temporal and spatial sequence of bone healing during DO remains poorly understood. A better understanding of the timing, location and degree of healing within the regenerate will be a key component in defining the overall quality of DO and may help to reveal the underlying mechanisms of mechanically induced new bone formation. Our specific aim is to determine the sequence of events leading to healing of the distraction regenerate while mapping the pattern of mineralization within the gap.
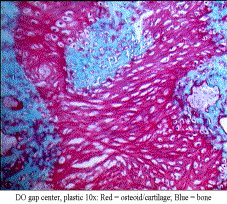
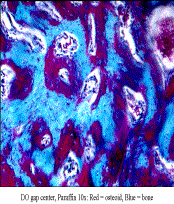
Methods: A modified Ilazerov-type external fixator was placed bilaterally in 400g male Sprague-Dawley rats. A unilateral vertical osteotomy was placed posterior to the 3rd molar. DO: 4-day latency with 5.1mm total gap and C: contra-lateral mandible as control. All animals underwent 28 days of consolidation. Calcein Green and Xylenol Orange were injected IP after latency (POD 5) and distraction (POD 13), respectively. Post-euthanasia, hemi-mandibles were fixed in 70% ETOH and stored away from light. Imaging: Using µCT (GE Healthcare Biosciences), mandibles were scanned at 45micron voxel size. Regions of interest (ROI) were created using anatomic landmarks and matched with prior templates, using beta test software version with advanced ROI abilities. Alpha-blend imaging was performed using 256 colorimetric grayscale. Histology: Plastic (PMMA) embedding into blocks by dehydration/infiltration under vacuum oven. I) Sagittal sections were cut (Exakt), micro-grinded to 200μm, polished and mounted. II) 5μm sagittal sections cut (Polycut), mounted, and then stained with modified Movat's, Gomori and Toluidine Blue for plastic. Additionally, 10μm paraffin sections (de-calcified) from prior group (identical surgical protocol) were stained with Gomori's trichrome. We used a smaller ROI on Bioquant® software, directly proportional to μCT, which only measured the central region, rather than the entire gap. Measures included tissue volume (TV); bone volume (BV) and osteoid volume (OV), then calculated BV/TV and OV/TV. Independent t-tests were used to compare DO parameters.
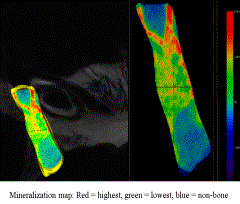
Results: DO histology revealed a centralized cartilage and osteoid intermediary, with inward growth of a canal-like pattern of woven bone. Surprisingly, we found islands of osteoid and bone scattered throughout the gap, rather than from the periphery. μCT alpha-blend revealed a similar scattered pattern of various low to high mineralization color peaks throughout the gap. Xylenol Orange supported this with a scattered fluorescence throughout the DO gap. The ratio of BV/TV compared to OV/TV ratio was not significantly different in our DO regenerate.
Conclusions: We have determined and outlined the sequence of healing within the distraction regenerate as well as created a μCT derived alpha-blend map of the gap. The degree of non-mineralized matrix to bone ratio within the center of the DO gap, despite clinical evidence of bony union, suggests deposition is ongoing and that scattered patterns of bone healing are integral to the mechanism of DO. The use of newer μCT algorithms mapping mineralization within the distraction gap is supported by light and fluorescent microscopy. Mapping the DO gap should improve our ability to evaluate the quality of bone healing within the regenerate and enable us to establish the most advantageous treatment protocols to promote the best possible formation of new bone.