Sunday, October 8, 2006
10900
Nonviral-Mediated Gene Therapy of Adipose-Derived Stem Cells
INTRODUCTION: Tissue regeneration strategies have included the delivery of recombinant proteins, viral-mediated and nonviral-mediated gene therapies. The high dosing requirements, expense, and safety issues have limited the potential of recombinant protein delivery. Safety issues continue to be a concern for viral-mediated gene therapy. Nonviral-mediated gene therapy continues to be limited by low transfection efficiencies. Enhancing nonviral-mediated gene therapy by improving uptake and expression is clinically attractive. For the present studies, several types of plasmid DNA-polymer complexes were evaluated in vitro for their ability to transfect and express enhanced green fluorescent protein (eGFP) in adipose-derived stem cells (ADSCs).
METHODS: The eGFP was spliced into a plasmid vector utilizing the elongation factor 1 alpha promoter with an enhanced CMV element (pCEF1a-DNT-eGFP) and purified by Mobius 1000 Plasmid Kits (Novagen, Madison, WI). Following the initial isolation procedure, ADSCs were plated at a seeding density of 25,000/well in 12-well plates (n=3). ADSC purity was evaulated with the putative ADSC marker CD49d at passage 2 and 3. Five plasmid DNA-polymer complexes, as well as controls, were exposed to the ADSCs for 24 hours. After a 3 day exposure period, the cells were removed by trypsin, washed and scanned for transfection efficiencies by a fluorescence-activated cell sorter (FACStar cell sorter with FACScan instrument, Becton-Dickinson). Cells expressing GFP are presented as a percentage of ADSCs expressing GFP vs. total cell number. Single-factor ANOVA with post-hoc analysis was used to determine statistical significance with p < 0.05 established as significant.
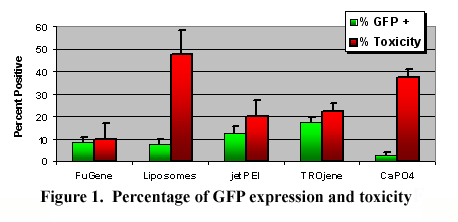
RESULTS: Initial characterization at P2 and P3 with the putative ADSC marker CD49d has exhibited purities of 73% and 68%, respectively, as determined by FACS analyses. The percentages of ADSCs expressing GFP at 3 days following the transfection procedures are illustrated in Figure 1. Overall, the variable configurations influenced GFP expression (p<0.001). The ADSCs exposed to the DNA:TROjene group expressed statistically more GFP than the other groups (p <0.05), with the exception of jetPEI. The FuGene reagent exhibited the lowest toxicity, while the liposomes and CaPO4 groups were consistently toxic.
CONCLUSION: The DNA:TROjene complex was the most effective nonviral-mediated vector complex for transfection of ADSCs in the present studies. The expression cassette from the present studies has been modified to contain and express VEGF and IGF-1. DNA:TROjene complexes with plasmid DNA expressing VEGF and IGF-1 are being evaluated to improve tissue-engineered implant vascularity and ADSC durability, respectively.
View Synopsis (.doc format, 86.0 kb)