Sunday, October 8, 2006
11058
CROSSEAL* Fibrin Sealant Provides an Enhanced Sustained Delivery System for Gene Uptake and Expression
INTRODUCTION: Some of the current bone regeneration strategies under investigation as alternatives to autografts, allogeneic banked bone, and other bone substitutes, include delivery of recombinant proteins, viral-mediated, and nonviral-mediated gene therapy. The high dosing requirements, expense, and safety issues have limited the potential of recombinant protein delivery. For bone regeneration, gene therapy is the delivery, uptake and expression of DNA that has been localized to a wound bed. Safety issues continue to be a concern for viral-mediated gene therapy. Nonviral-mediated gene therapy is limited by low transfection efficiencies. Enhancing nonviral-mediated gene therapy by improving uptake and expression is clinically attractive. The present studies evaluated the use of a sustained delivery system of a condensed circular DNA plasmid encoding green fluorescent protein (GFP) within a polycation complex, jetPEI, contained with the human fibrin sealant, CROSSEAL*. This solution was infiltrated into circular polylactic acid (PLA) porous scaffolds and placed on cell cultures in vitro to assess transfection efficiencies at a 2-, 4-, and 7-day interval.
METHODS: Plasmid DNA expressing an enhanced green fluorescent protein (eGFP) reporter gene was condensed within a polycation complex, jetPEI (MP Biomedicals, Irvive, CA), mixed with the human fibrin sealant, CROSSEAL* (Johnson & Johnson, Somerville, NJ), and loaded within 8 mm circular polylactic acid (PLA) porous scaffolds. These complexes, along with controls and a GFP:FuGene 6 (Roche Biomolecular, Indianapolis, IN) group, were evaluated in vitro for their ability to transfect and express GFP into BHK cells (baby hamster kidney fibroblasts) at a 2-, 4-, and 7-day interval following transfer into fresh 12-well plates.
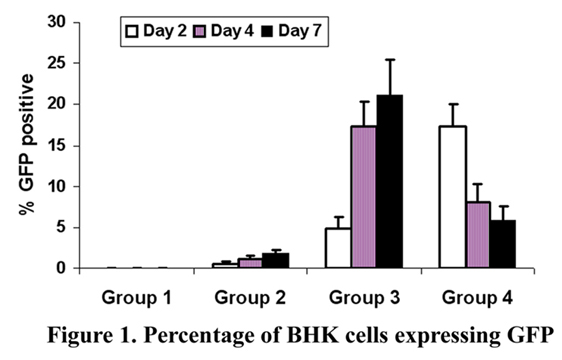
Group 1: CROSSEAL*/PLA (negative control);
Group 2: GFP in CROSSEAL*/PLA;
Group 3: GFP:jetPEI in CROSSEAL*/PLA;
Group 4: GFP:FuGene 6 in CROSSEAL*/PLA.
Thereafter, transfection efficiencies were determined by a FACScan instrument (Becton-Dickinson). Data are presented as mean % +/- SD of cells expressing GFP vs. total cell number. Statistical analyses were conducted using single-factor ANOVA and Fisher's PLSD post hoc analysis with significance established at p < 0.05.
RESULTS: The mean percent of BHK cells expressing GFP for the negative control was 0+/-0% for all negative control samples. Group 2 (GFP in CROSSEAL*/PLA) exhibited transfection efficiences of 0.6 (+/-0.2), 1.2 (+/-0.3), 1.9 (+/-0.4), at the 2-, 4-, and 7-day intervals, respectively. Groups 3 and 4 demonstrated transfection efficiences of 4.9(+/-1.4), 17.4(+/-2.7); and 16.1(+/-2.9), 8.1(+/-2.1); and 21.2(+/-4.2), (5.9(+/-1.6); at the 2-, 4-, and 7-day intervals, respectively. Significance was established between all groups over the negative control and Group 3 and 4 over Groups 1 and 2. Group 4 exhibited the highest transfection efficiency at the 2-day interval, while Group 3 exhibited the highest transfection efficiencies at the 4-, and 7-day intervals. Data are presented in Figure 1.
CONCLUSION: The results from the present studies evaluated the impact of moving the DNA:polymer complexes delivered from the circular porous PLA scaffolds to new wells after 2-, 4-, and 7-day intervals. Although Group 4, GFP:FuGene 6 in CROSSEAL*/PLA, exhibited the highest transfection efficiency at the 2-day interval, Group 3, the GFP:jetPEI in CROSSEAL*/PLA, exhibited enhanced output and expression at the 4-, and 7-day intervals. The combination of the GFP:jetPEI in CROSSEAL* appears to improve the functional stability and release duration of incorporated DNA-polymer complexes in the present in vitro studies. These results have improved on our previous studies evaluating resorbable gene-activated matrix formulations. Future studies will evaluate outcomes with the present configurations in standard animal models of bone regeneration.
View Synopsis (.doc format, 31.0 kb)