Tuesday, October 10, 2006
10359
Collagenase Injection in the Treatment of Cellulite
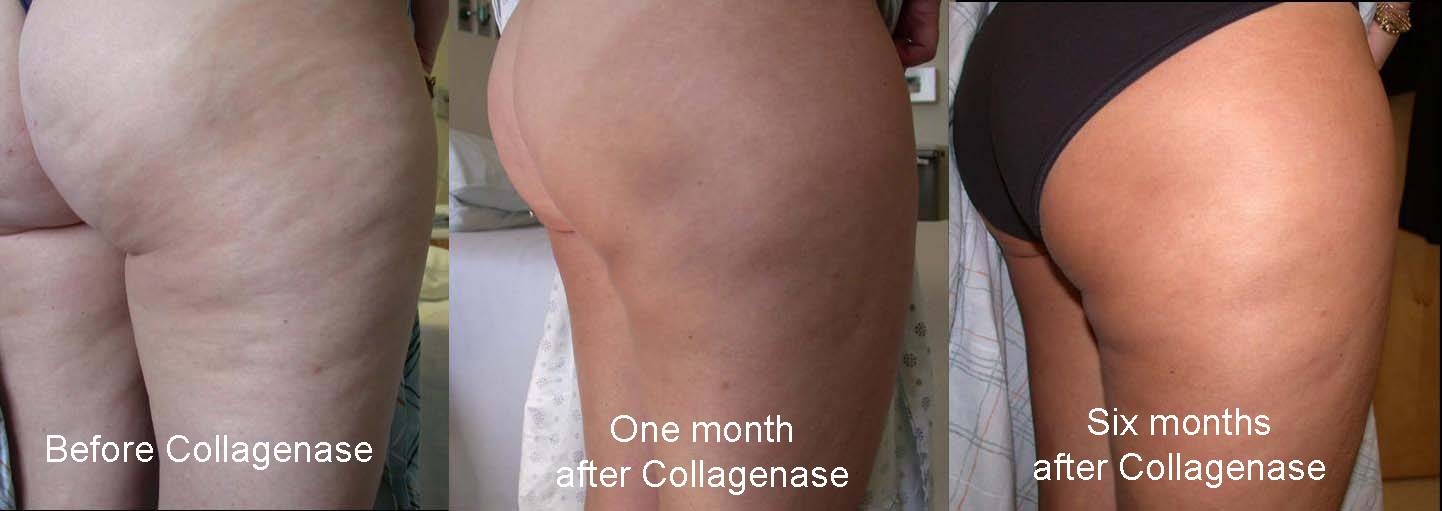
There remains no effective treatment for dimpling of the thighs and buttock skin known as cellulite. We postulated that if the anchoring connective tissue septae and/or hypertrophied subcutaneous adipose tissue could be lysed by collagenase that the contour irregularity would be improved.The purpose of this Phase 2,FDA study was to test the safety and efficacy of collagenase injection as a treatment for cellulite. Methods:10 female patients with a mean BMI of 28,avg. age 41 enrolled. 0.58mg of collagenase was injected into the posterolateral thigh. Patients were followed and photographed post injection and the area of cellulite quantified in cm. Results:There was a significant reduction in cellulite appearance of the injected area. Cellulite area was reduced by 77% day 1, 74% at 1 week,89% at 1 month,86% at 3 mos.,76% at 6 mons.Patient satisfaction was 1.75 at 6 mons(1= completely satisfied, 4= not satisfied).BMI and thigh circumference showed no significant differences from baseline values. Side effects included injection area soreness, ecchymosis and mild edema which resolved in a mean of 10, 18 and 6 days, respectively. Conclusions: This study has shown that collagenase injection for cellulite is safe and has merit.This therapy warrants continued testing within the FDA regulatory process.
View Synopsis (.doc format, 240.0 kb)
