Sunday, October 8, 2006
11097
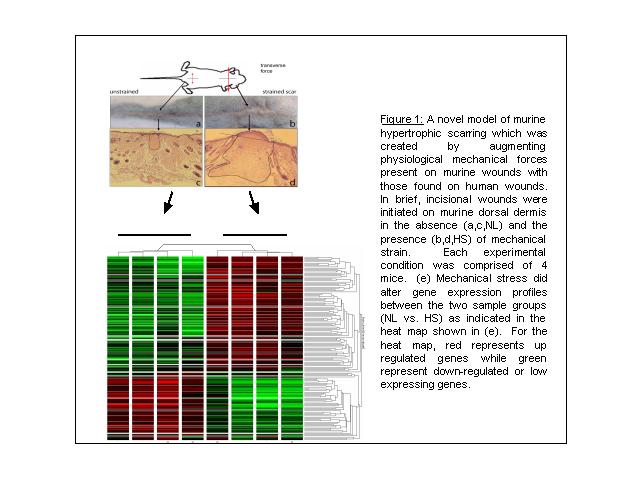
Microarray Profile for Murine Models of Hypertrophic Scars
In our presentation, we propose to assess the microarray gene expression profile of a murine model for hypertrophic scars. We hypothesized that the expression profile would be of a pro-proliferative, pro-angiogenic but anti-apoptotic nature and that mechanical strain serves as a main impetus. To test this hypothesis in vivo, dorsal skin of mice was subjected to two conditions: (i) tissue given incisional wound alone (NL) and (ii) tissue given both mechanical stretch and incisional wounding (HS) (n=4 mice in each group, Figure1a-d). After 11 days of treatment, total RNA was harvested from skin tissue and hybridized to Affymetrix 43K 2.0 GeneChips. Using the permutation-based algorithm Significance Analysis of Microarrays [1], we identified 347 genes that reproducibly distinguished incisional wound versus incision plus mechanical stress (false discovery rate < 0.05). As shown in Fig. 1e, mechanical stress induced the expression genes involved the extra cellular matrix synthesis (asporin, laminin B, procollagen or collagen types III-VII, lysyl oxidase). Hypertrophic scars are known to over express matrix-associated proteins; thus these results thus support a role for mechanical stress in inducing scar hypertrophy. Expression of several genes related to angiogenesis (lysyl oxidase, VCAM-1, Angiopoietin-like 2 protein, RAMP2 or adrenomedullin receptor) were also significantly induced by mechanical stress, thus pointing to the possible involvement of altered neovascularization in the HS phenotype. Finally, multiple growth factors (IGF1, Bdnf, Osf2, Raf53, TFPI, Lef1, Csf3r), signal transducers (Vav, c-fes, creatine kinase, Ste20, Nek7, Dcamk1, Macs, and Eif2ak3) and transcription factors (HIF-1á, c-maf, Tcf4, MITF4, Tert2ip, Mafb) associated with cellular proliferation and differentiation were induced by mechanical stress. These results confirm that hypertrophic scars represent an activated, pro-proliferative state. Moreover, the activation of HIF-1á suggests a confluence of mechanical stress with hypoxia-regulated pathways to induce scarring. In summary, these preliminary data indicate the feasibility of using animal models of mechanical stress and gene expression profiling to identify molecular pathways that may be dysregulated in hypertrophic scarring, thus directing subsequent functional analyses
1. Tusher, V.G., R. Tibshirani, and G. Chu, Significance analysis of microarrays applied to the ionizing radiation response. Proc. Natl. Acad. Sci., 2001. 98(9): p. 5116-5121.