Tuesday, November 4, 2008
14496
In Vivo Electrophysiologic Properties of Poly 3,4-ethylenedioxythiophene (PEDOT) in a Biosynthetic Nerve Interface
Introduction:
Extremity amputation causes substantial functional deficits and reductions in tactile interactions with the environment while performing activities of daily living. Restoring mechanical and sensory function following amputation is critically important. Robotic prosthetics can restore many of the necessary functions, yet a high fidelity interface with cortical or peripheral signals remains elusive. Existing capacitive and myoelectrical peripheral interface devices have poor durability and fidelity for detecting small specialized physiologic currents. Biointegrated neural prosthetic interfaces must address the conversion of physiologic, ion current derived action potentials (<1mA) to electron mass-transport currents for device and electronics circuitry. One of our aims is developing a durable, high-fidelity, biologically integrated neural prosthetic interface that will detect peripheral nervous system (PNS) motor signals allowing robotic prosthetics control. In this study, we evaluate in vivo conductive properties of a bioartifical PNS interface utilizing poly 3,4-ethylenedioxythiophene (PEDOT), a novel, highly electroconductive, biocompatible compound polymerized on a chemically acellularized muscle scaffold.
Methods:
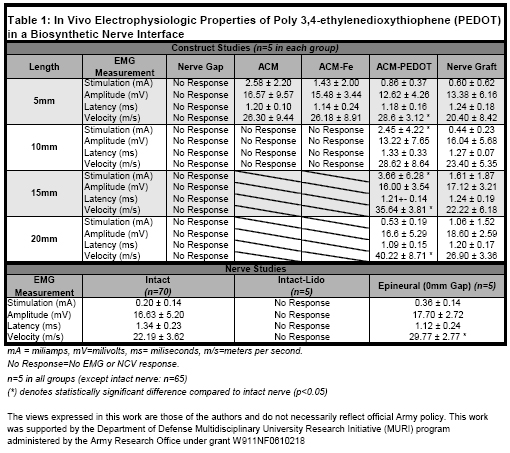
A rat (Fischer Scientficic F344) hindlimb peroneal nerve model was used for all experiments. We tested several biomaterials ability to detect and propagate an efferent action potential across a nerve gap, and stimulate a corresponding action potential in the distal peroneal nerve in the acute setting. The purpose of these experiments was specifically determine if the electroconductive polymer, PEDOT, could detect and stimulate physiologic action potentials for use as a PNS interface, not to design a biomaterial to be used as a nerve graft clinically. Three artificial, biologic, and bioartificial constructs were evaluated in a peroneal nerve gap model: 1) Chemically acellularized rat muscle (ACM), 2) Acellular muscle treated with Iron (III) Chloride, a PEDOT polymerization reagent (ACM-Fe), and 3) Acellular muscle treated with a PEDOT chemical polymerization process (ACM-PEDOT). These materials do not possess cellular machinery necessary for action potential propagation and presumably conduct via electron mass transport. Control groups for these experiments included: 1) Intact peroneal nerve (Intact) (N=70), 2) Intact nerve in presence of lidocaine (to confirm stimulator derived action potential versus electron current) (Intact-Lido), 3) a single coaptaion epineural repair (Epineural) 4) Nerve gaps with no graft for reconstruction (Nerve Gap), and 4) Autogenous nerve (Nerve Graft). N=5 for each experimental group. Peroneal nerve gap lengths were 0,5,10,15 and 20mm. All nerve and material coaptations were performed with 10-0 nylon suture using a standard epineural suture technique. We evaluated the electrophysiologic properties in each experimental group by exogenously stimulating the peroneal nerve proximal to the coaptation site in vivo and measuring distal to the distal coaptation site using standard NCV/EMG techniques. Stimulation amperage (mA) was varied to obtain maximal EMG response (mV) in the extensor digitorum longus (EDL) muscle, with measurement of the associated nerve conduction latency (ms) and velocity (m/s).
Results:
Exogenous nerve stimulation proximally in the intact peroneal nerve gerenerated typical action potentials distally; this response was eliminated when voltage gated sodium channels were blocked by lidocaine. NCV/EMG results (Table 1) demonstrate ACM-PEDOT constructs conduct physiologic currents (0.53 ± 0.19 mA) up to 20mm with maximal amplitude of 16.60 ± 5.29 mV, and latency of 1.09 ± 0.15 ms. These ACM-PEDOT data are consistent with NCV/EMG values for intact nerve or from similar length nerve autografts. ACM-PEDOT constructs showed a statistical increase in conductive velocity (40.22 ± 8.71 m/s) compared with intact nerve (22.15 ± 3.68 m/s). 10mm ACM and ACM-Fe constructs were non-conductive and thus, longer lengths were not tested.
Conclusions:
PEDOT coated acellular muscle constructs can transmit the small currents (<1 mA) associated with physiologic action potentials in vivo. This indicates that the proximal nerve action potential can be electrically "sensed" via a PEDOT construct. Given the physiologically compatible conductive properties of this novel biomaterial, we have initiated development of implantable, long term, integrated bioelectrical coupler between the proximal nerve segment and the PEDOT construct, and between the PEDOT construct and electronics, with the ultimate goal of providing appropriate afferent control to a prosthetic limb.
See more of Hand, Upper Extremity/Microsurgery
Back to 2008am Complete Scientific Program
