Sunday, November 2, 2008
14726
Hydrogen Sulfide Significantly Mitigates Ischemia Reperfusion Injury
Hydrogen Sulfide Significantly Mitigates Ischemia Reperfusion Injury
Sunil P. Singh, BA, Daniel C. Rafii, BA, David C. Lyden, MD, PhD, Jason A. Spector, MD
Background: Despite the ubiquity of microvascular free tissue transfer, a major limitation and source of morbidity in these procedures is related to reperfusion injury of the transferred tissue. Longer periods of ischemia may result in significant reperfusion injury ultimately leading to partial flap loss or flap failure. Hydrogen sulfide, though traditionally considered a toxic species, has recently been recognized as an important endogenous signaling molecule with potential as a cytoprotectant. Our unique approach to improving flap tolerance to ischemia induces a “suspended animation” like state within the flap tissue by exposure to Hydrogen Sulfide. This compound is thought to bind to cytochrome c oxidase and reversibly interrupt the process of oxidative phosphorylation. Because muscle and intestine are known to be among the most sensitive flap tissues to ischemia/reperfusion injury, we chose to establish the validity of our hypothesis using these tissue types.
Methods: Our study assessed the ability of H2S to mitigate ischemia reperfusion in 2 different tissue types; intestine and muscle. Ten 8 week old wildtype (C57/b6) male mice were exposed to either a mixture of H2S (150ppm) balanced with room air for 30 minutes (6 animals) or room air alone for 30 minutes (6 animals). After H2S or room air (control) exposure, intestinal blood supply was occluded for 15 minutes, 2 hours or 4 hours and finally all groups were allowed to reperfuse for 3 hours. Intestinal tissue was then harvested. Paraffin sections were stained with Hematoxylin and Eosin (H&E) and TUNEL assay antibody. Additionally, twelve 8 week old wildtype (C57/b6) male mice were exposed to either a mixture of H2S (150ppm) balanced with room air for 30 minutes (6 animals) or room air alone for 30 minutes (6 animals). Hind limb ischemia was induced by tourniquet applied above the greater trochanter for a period of 3 hours followed by 3 hours reperfusion. The contralateral leg served as a control. Gastrocnemius and soleus muscles were harvested bilaterally. Frozen sections were stained with H&E and TUNEL assay antibody.
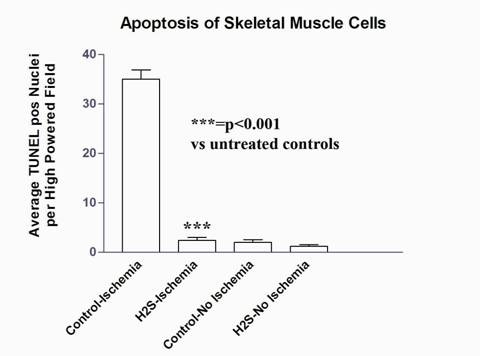
H&E sections were examined for breakdown of villus architecture (intestine) and breakdown of muscular architecture (vacuole formation, edema, fiber separation). Quantitative analysis of apoptosis for both intestine and muscle tissue was performed with a TUNEL Assay as an average of TUNEL positive nuclei per high powered field across five fields per slide (Fig 1-2). Statistical significance was determined by ANOVA single factor with Tukey-Kramer post-hoc pair testing. All experiments were performed in triplicate.
Results:
Intestinal tissue treated with H2S demonstrated a significant reduction in the number of apoptotic cells versus control. This cytoprotective effect was noted after 15 minutes and 2 hours of ischemia (92%, 91% reduction in the number of apoptotic cells versus untreated controls, respectively). After 4 hours of ischemia, the protective effect was less evident (38% reduction) (Fig 1). H&E staining of intestinal sections from H2S treated animals showed a preservation of intestinal villous architecture versus controls. As in the TUNEL staining, this difference was most evident after 2 hours of ischemia. Muscle tissue also showed significant reduction in apoptosis versus controls with a preservation of muscular architecture and significantly reduced number of apoptotic cells (93%) in treated ischemic groups versus ischemic controls (Fig 2). There were no significant differences in numbers of apoptotic cells between non-ischemic muscle treated with H2S versus control.
Conclusion: This study demonstrated a significant protective effect through gaseous hydrogen sulfide exposure to intestinal and muscle tissue against ischemia reperfusion injury. These data suggest that hydrogen sulfide exposure mitigates ischemia-reperfusion injury without direct cellular toxicity in our target range, giving this molecule significant therapeutic potential as a cytoprotectant in free tissue transfer and other conditions associated with ischemic conditions.
Figure 1:
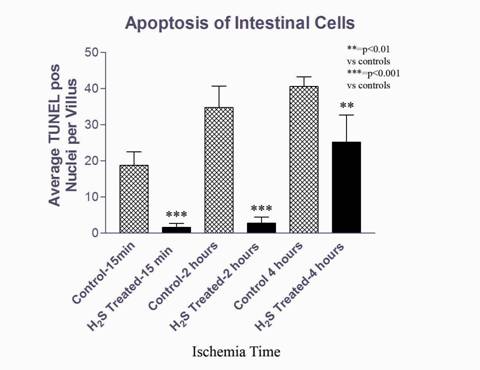
Figure 2: