Friday, February 1, 2008
13865
Delivery of targeted chemotherapy via genetically modified microvascular flaps suppresses primary tumor growth
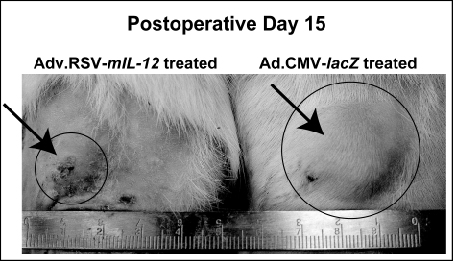
Introduction: Two obstacles limiting the application of gene therapy to solid tumors are the inability to target gene delivery and the risk of host immune response to the vector. We have previously described a technique to selectively transduce microvascular free flaps (MVFF) during the ex vivo period, surgically targeting transgene expression and minimizing systemic toxicity. Microvascular free flaps are widely used for cancer reconstruction, but have not been previously examined as a peptide delivery system. The present study demonstrated for the first time that microvascular free flaps can be transduced ex vivo to express biologically active molecules [murine interleukin-12 (mIL-12)] that suppress primary tumor growth of a rodent breast adenocarcinoma cell line (MADB106). Methods: Superficial inferior epigastric flaps were dissected in male rats. During the ex vivo period, the MVFF were perfused with either 1 x 1010 ifu of Ad.RSV-mIL-12 (n=10) or Ad.CMV-lacZ vector (n=10) via the pedicle with the efferent vein clamped. Following a one hour incubation period, the efferent vein was unclamped and the flap was flushed to remove any unincorporated viral particles. After reanastomosis, 1 x 106 MADB106 cells stably transfected with â-hCG were injected subcutaneously adjacent to the flap. Tumor burden was assessed by both indirect and direct measurements. Serum was collected at the time of surgery and every third day thereafter and analyzed for â-hCG. Direct measurement of the tumor with dial-calipers was performed at the same time serum was collected. IL-12 expression was quantified in the flap, peri-flap tissue and pedicle from animals sacrificed at days 7, 14, 21 and 28 (n=4 each group) using a murine ELISA kit. Immunohistologic analysis was performed on frozen sections of tumors from the IL-12-treated and control-treated groups at day14. Antibodies directed against the CD8+ and CD161+ antigens were used to determine whether IL-12 treatment promoted the accumulation of inflammatory leukocytes within the tumors. Since IL-12 has been shown to promote IFN-ã expression in T cells and natural killer cells, levels of IFN-ã were quantified in tumor tissue from treated and control animals by ELISA. Systemic toxicity was evaluated by measuring liver enzyme function and by histologic examination of distant organs for inflammatory changes. To further demonstrate that our ex vivo approach minimized systemic toxicity compared to other methods of gene delivery, we evaluated the effects of systemic (in vivo) Ad.RSV-mIL-12 administration by tail vein injection to our current ex vivo approach. Results: In animals treated with Ad.RSV-mIL-12, we observed a 79% reduction in tumor volume as compared control rats (P<0.05). Differences in serum â-hCG were also observed at day 9 (p<0.001). IL-12 expression from the flap, peri-flap tissue and pedicle was confirmed by ELISA. The mechanism by which IL-12 suppresses tumor growth was also demonstrated. Tumors from IL-12 treated animals exhibited a heavy infiltration CD8+ and CD161 cells and showed a 4.5 fold increase in IFN-ã expression when compared to controls. There was no observed difference in liver enzymes levels and normal liver histology was preserved in the two groups. In animals who underwent systemic in vivo viral transduction via tail vein injection, serum transaminase levels were significantly higher than the ex vivo-treated animals and histologic examination of the liver revealed inflammatory changes with mononuclear cell infiltration. Conclusion: This study demonstrated that microvascular free flaps can be genetically modified during the ex vivo period to locally express chemotherapeutic proteins and suppress tumor growth while minimizing systemic toxicity. Our technique allows biologic therapy to be precisely targeted and avoids many of the practical obstacles currently limiting gene therapy. Microvascular free flaps currently serve only a reconstructive role in the treatment of cancer. Since free tissue transfer is routinely performed following the resection of solid tumors, this represents an unfulfilled opportunity to locally deliver chemotherapeutic transgenes to patients as an adjuvant therapy following oncologic surgery.