Sunday, October 3, 2010
17883
Controlled Neurotrophic Factor Delivery to Promote Functional Peripheral Nerve Regeneration
Purpose:
Despite advances in the treatment of peripheral nerve injury, functional recovery is often suboptimal. Alternatives to current treatments (autografts) have included nerve guidance conduits. Glial-derived neurotrophic factor (GDNF) is the most potent motor growth factor to date and may enhance regenerating motor axons. Therefore, the delivery of this growth factor from a nerve guidance conduit may enhance motor nerve regeneration and functional recovery. We chose to investigate the use of an affinity-based delivery system (DS) constructed in fibrin (Figure 1) to provide controlled local delivery of growth factor to the injury site to enhance motor nerve regeneration.
Methods:
We evaluated the effect of controlled delivery of GDNF from fibrin-filled nerve guidance conduits on motor nerve regeneration and functional recovery in a 13 mm rat sciatic nerve defect. Seven experimental groups were evaluated including: GDNF and DS and NGF with DS and control groups including: GDNF without a DS, isograft (positive control), fibrin with growth factors and empty conduits. Animals were monitored monthly for behavioral recovery, which included sciatic functional index and grid-grip abilities, and analyzed after 12 weeks for muscle contractile force assessment and retrograde labeling of motor neurons.
Results:
Groups with growth factor in the nerve guidance conduit performed similar to or better (GDNF) than the isograft in behavioral tests and had similar spared relative muscle mass (53 – 63%). Additionally, groups with controlled GDNF delivery had greater extensor digitorum longus contractile force than the isograft (56% compared to 25%, normalized to uninjured muscle). Motor regeneration assessed by retrograde labeling revealed that controlled GDNF delivery matched the isograft in the number of regenerating normalized ventral horn neurons (~60%) (Figure 2).
Conclusion:
Overall, the controlled delivery of GDNF from a nerve guidance conduit demonstrated improved measures of functional recovery, suggesting GDNF has potential as a treatment for motor nerve injury.
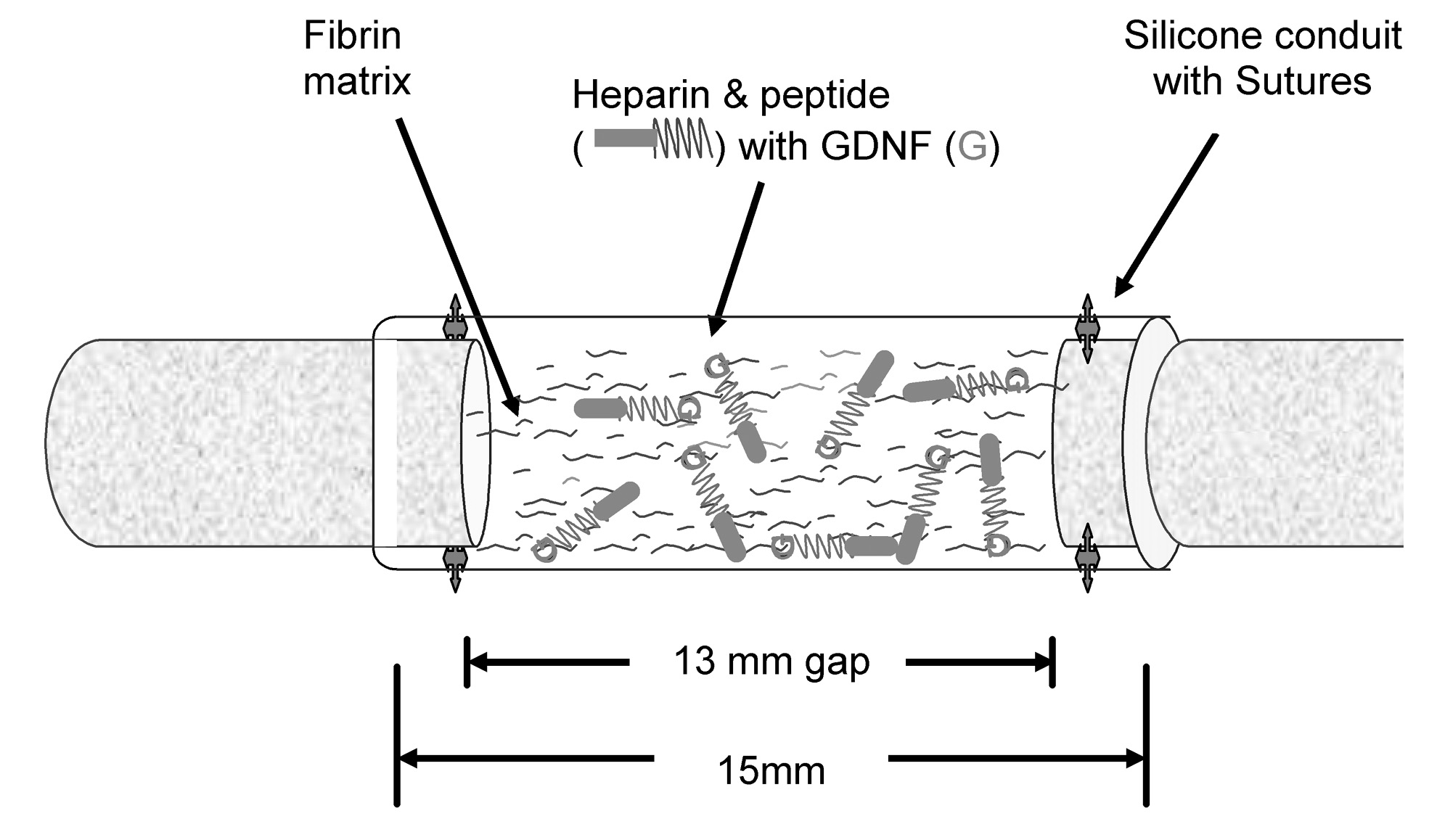
Figure 1 Schematic representation of surgical implantation of nerve guidance conduit containing the affinity-based delivery system (DS) (modified from Wood et al.).

Figure 2 Normalized ventral horn neurons retrograde labeled 12 weeks after injury.
References:
- Wood MD, Moore AM, Hunter DA, Tuffaha S, Borschel GH, Mackinnon SE, and Sakiyama-Elbert SE. Affinity-based release of glial-derived neurotrophic factor from fibrin matrices enhances sciatic nerve regeneration. Acta Biomaterialia 5:959-968, 2009.
