Room 2 (Henry B. Gonzalez Convention Center)
Sunday, November 3, 2002
8:00 AM - 4:00 PM
Room 2 (Henry B. Gonzalez Convention Center)
Monday, November 4, 2002
8:00 AM - 4:00 PM
Room 2 (Henry B. Gonzalez Convention Center)
Tuesday, November 5, 2002
8:00 AM - 4:00 PM
Room 2 (Henry B. Gonzalez Convention Center)
Wednesday, November 6, 2002
8:00 AM - 4:00 PM
817
P43 - Pyoderma Gangrenosum Developing After Throidectomy
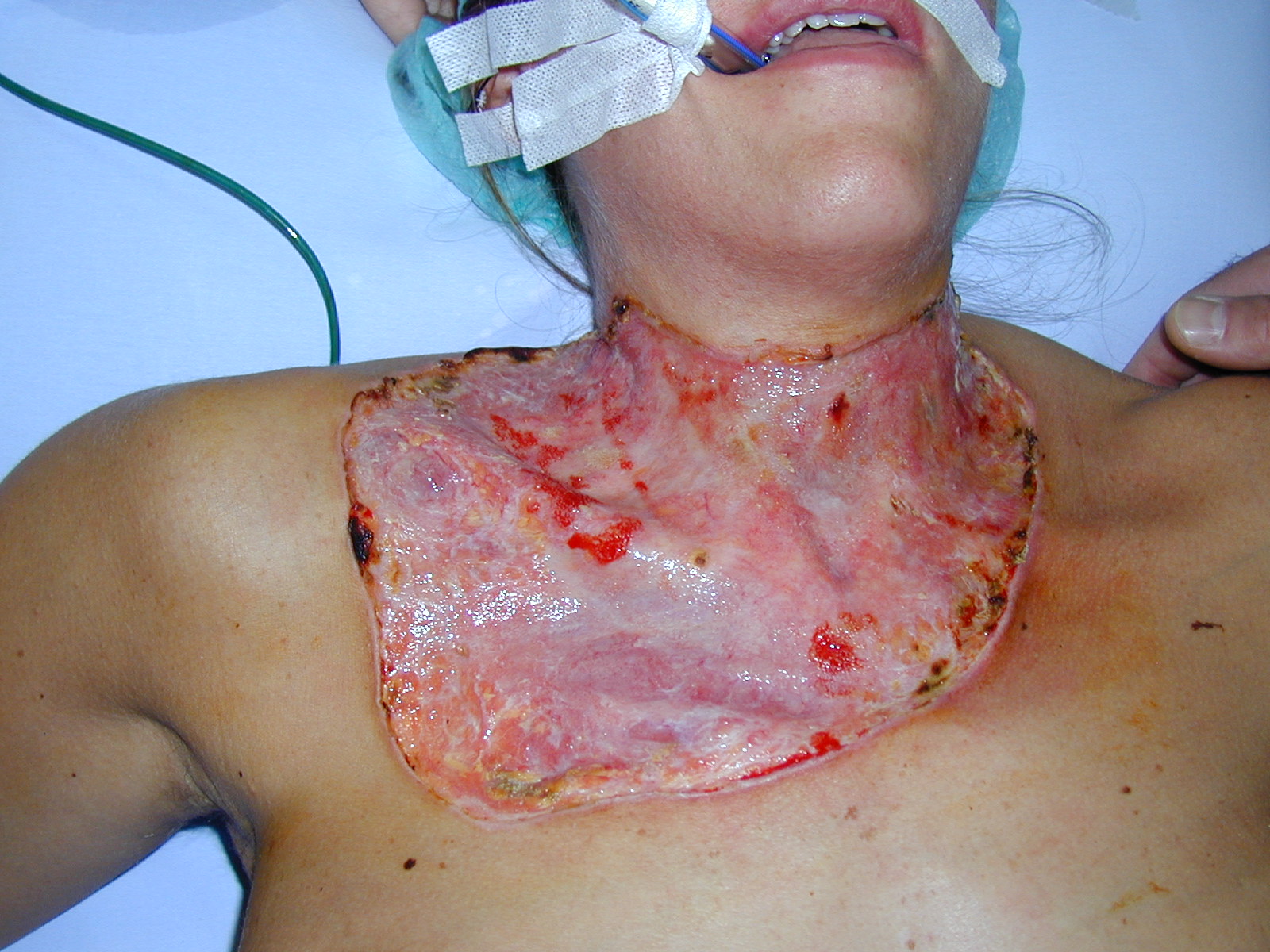
Pyoderma gangrenosum (PG) is a rare, destructive, necrotizing ulcerations with a rolled vegetating border. It has been classified into 4 distinct, clinical, and histologic variants-ulcerative, pustular, bullous and vegetative forms. PG can occur spontaneously or can be associated with a systemic disease. In rare instances it may develop after surgical operations or minor injuries causing traumatization of the skin. The cause of PG is unknown, but immune surveillance abnormalities, including both humoral and cell-mediated defects, has been suggested. Defects in cellmediated immunity and in polymorphonuclear leukocyte function have been described. None of the current, mostly immunosuppressive, therapies are entirely effective. Because of pathergy, many clinicians avoid managing these nonhealing ulcers with aggressive surgical debridement and autologous skin grafts. We report a 29-year-old previously healthy woman presented with an ulcerative form of PG begins as vesicopustules and tender erythematous nodules that rapidly necrotize to form large irregular ulcers with bluish, undermined edges with pathergy immediately after throidectomy at the lower site of the surgical incision on the cervical region, and disseminated sternal and upper anterior thoracal area. The ulcer persisted and rapidly enlarged during the next few days. Lesion arised on her collar, upper sternal and upper right thoracal area on the lower side of her cervical incision side. The clinical and histopathologic findings were consistent with PG. An extensive investigations including complete blood cell count, liver and renal function tests, serum protein electrophoresis, antinuclear antibody, quantitative immunoglobulins, rheumatoid factor, VDRL, HIV, hepatitis B testing, abdominal ultrasound, and wound cultures were noncontributory. Colonoscopy were negative for Crohn’s disease or ulcerative colitis. Dissemination of the ulcer did not respond to topical therapies but responded well to oral prednisone ( 80 mg/d ) and oral cyclosporin A (4 mg/kg per day) and was eventually repaired by skin grafting. During the 2 months, the prednisone dose had been gradually reduced to 10 mg/d and cyclosporin A (1 mg/kg per day), thereby decreasing the risk of nephrotoxicity, hypertension, hepatotoxicity and other side effects of the drug. By the 16th-week visit, cyclosporin was discontinued. DISCUSSION Treatment of PG has focused on the identification and control of the underlying systemic disease and immunosuppression and treatment of secondary infection. The ideal mode of therapy for PG varies according to the type and severity of the disease presentation. The course of the disease is unpredictable and the response to therapy variable from patient to patient. For patients with ulcers that are rapidly progressive, prompt control of the process is essential to provide pain relief, avoid secondary bacterial infections, and minimize the degree of scarring. Because of cyclosporine’s proven efficacy and rapid response, many have advocated its use earlier in the course of the disease as first-line theraphy. We propose that the application of split thickness skin graft (STSG) accelerates wound healing and decreases the risks of side effects (renal failure, hypertension, hepatotoxicity, seizures, and the development of lymphomas) from long term maintenance therapy with immunosupressive agents. No report exists about postoperative PG after throidectomy. We reported an effective treatment of one case of rapidly progressive PG immediately after thyroidectomy with an application of STSG to the defect, provided immediate pain relief and healing.
Fig 1. Anterior chestwall skin defect before treatment with skin grafting
Fig 2. Complete healing 4 months after skin grafting with minimal scar contracture.
View Synopsis (.doc format, 397.0 kb)
See more of Posters
Back to 2002 Complete Scientific Program
Back to 2002 Meeting home
