Sunday, October 10, 2004
5771
Characterization and Clinical Application of Tissue-Engineered Human Oral Mucosa Equivalent Based on Acellular Allogenic Dermal Matrix
[Introduction] Tissue-engineered skin equivalents composed of epidermal and dermal component have been widely used for the coverage of full-thickness skin defects. Oral mucosal keratinocytes have been introduced as an alternative source of cultured keratinocytes for the treatment of mucosal defects and extensive deep burns. We developed a tissue-engineered human oral mucosa equivalent based on acellular allogenic dermal matrix (ADM) and investigated its biological characteristics. Also, clinical application was performed in one case with the approval of ethical committee of Kyorin University and the patient's consent.
[Method] Human oral mucosal keratinocytes were separated from a piece of oral mucosa by serial enzymatic cell suspension using dispase and trypsin. Cells were cultured in a chemically-defined, bovine pituitary extract(BPE)-free medium(Defined keratinocyte SFM®, Gibco) and expanded through several passages until sufficient population of keratinocytes were obtained. The keratinocytes were then seeded onto an ADM which were prepared from cryopreserved human cadaveric skin by removing epidermis and cellular components of dermis. The composite was then cultured at an air-liquid interface for 4 days to promote stratification.
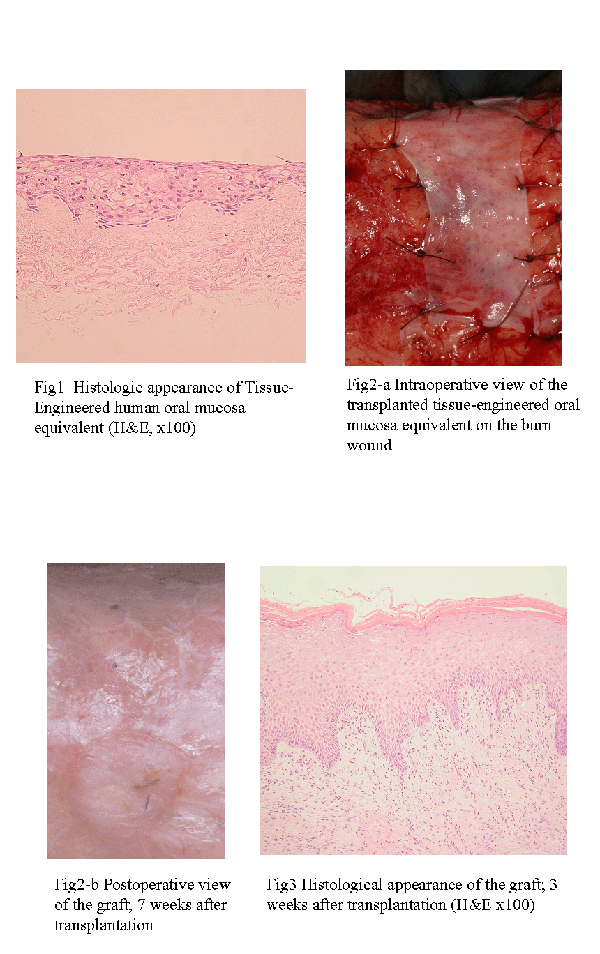
[Result] Histological study showed that the tissue-engineered mucosa equivalent had a well-stratified epithelial layer and rete ridge structure (fig1). Immunohistochemical staining of b1 integrin, involucrin and type‡W collagenshowed similarity to those of normal oral mucosa.
[Clinical application] We applied tissue-engineered human oral mucosa equivalents to one case of extensive burn. A piece of oral mucosa was harvested from the patient and autologous keratinocytes were cultured. Three weeks after the primary culture, the equivalent was prepared and transplanted on debrided full-thickness burn wounds of the calf (fig2-a). Approximately 30% of the graft survived and epithelized 7 weeks after the transplantation (fig2-b). The low survival rate was thought to be mainly due to local infection and sepsis. Histological examination of the graft showed well-stratified keratinocytes with marked parakeratosis(fig3).
[Discussion] The characteristics of our tissue-engineered oral mucosa equivalent are as follows; 1) Use of autologous oral mucosal keratinocytes as epidermal component. Oral mucosal keratinocytes have such advantages compared to epidermal keratinocytes as follows; a) higher proliferation rate b) lower rate of terminal differentiation. By these characteristics of oral mucosal keratinocytes, the equivalent can be produced in shorter time and emergent use such as extensive burn treatment is possible. 2) Use of ADM as a scaffold of dermal component ADM has a unaltered collagen matrix and intact basement membrane. These are favorable features for ingrowth of angiogenic cells and fibroblasts. 3) Safer method in keratinocyte culture There have been established several kinds of methods of culturing keratinocytes. Among them, Green's method which uses 3T3 feeder layer and Boyce's method which uses BPE have been common. Recently, however, more chemically-defined system is required for clinical application in order to prevent disease transmission such as prions. We used a chemically-defined medium which contains several kinds of growth factors instead of BPE to reduce the risk of disease transmission.
[Conclusion] We developed tissue-engineered human oral mucosa equivalent based on ADM, which showed histologically similar structure to the normal oral mucosa. It was suggested that this equivalent could become one of the surgical therapeutic choice for mucosal and skin defects.
View Synopsis (.doc format, 201.0 kb)