Sunday, October 10, 2004
6180
Synthesis Of Artificial Dermal Flap By Dilating Choke Vessels: Angiography And Lasergraphy In Rat Model
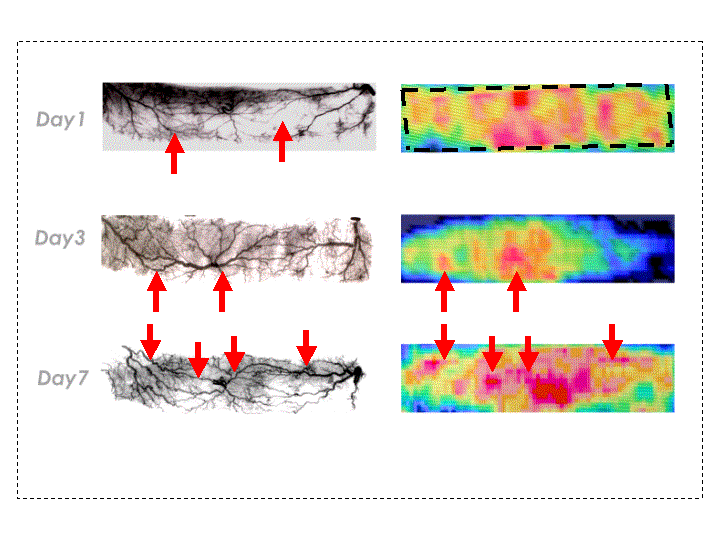
INTRODUCTION: Recently, various methods of creating prefabricated flaps using artificial dermis were described by several authors1,2). In their experimental models, however, dominant vascular bundles such as the saphenous vessels were needed and it took several weeks to vascularlize artificial dermis. The aim of this study was to synthesize the dominant axial vessel from the choke vessels, which are the tenuous anastomosing vessels in the skin and the subcutaneous tissues, and simultaneously vascularlize the artificial dermis, therefore, to generate the living artificial dermal flap. METHODS: On the back of the Wistar rat (male, 200g, 30 animals), a 2 x 8 cm rectangle skin flap, which contains three even vascular territories (thoracodorasal artery: TD, intercostal artery: IC and deep circumflex iliac artery: DCI), was elevated as a bipedicled island flap on TD and DCI. The artificial dermis (2 x 8cm) composed of collagen sponge and silicone membrane (PelnacTM, Gunze Co. Kyoto, Japan) put on the deep surface of the skin (panniculus carnosus) and sutured back in place. On the day 1, 3, and 7, the flaps were elevated again and converted to monopedicled flaps by severing DCI pedicle. Each artificial dermis and skin flap complex was served to the laser flowgraphy (L-MAP10TM, M and M Co. Tokyo, Japan) to observe the real time 2-dimentional blood flow. Each complex was injected with radiopaque agent (Microfil TM, FlowTek.Inc USA) for the angiography after the animals were euthanized. RESULTS: The laser flowgraphy in comparison with the angiography (Fig.) revealed the time sequential vascularlization of the artificial dermis according to the dilation of the choke vessels proceeding. On the day 1, the choke vessels dilated slightly on the angiogram (arrows), however, the changes were masked by the overlying artificial dermis (dotted line on the laserflowgram) because the vascularlization into the artificial dermis was immature. On the day 3, the caliber of the dilated choke vessel (arrows, angiogram) was large enough to be displayed as the high blood flow part in the flap (arrows, laserflowgram). On the day 7, the dilated choke vessels (arrows, angiogram) interconnected three pedicles (TD, IC, and DCI) to form one axialized vascular territory providing high blood flow axis in the artificial dermis (arrows, laserflowgram). CONCLUSION: The vascularlized artificial dermal flap by this method did not need sacrificing large vessels or skin in the autologous tissue donor site. The period needed for synthesis was so shorter than other methods that this flap could provide a good lining for burn or pressure sores, even if there is no optimal donor site. References 1) Y.Tanaka, K.C.Sung, A.Tsutsumi, et al. Tissue Engineering Skin Flaps: Which Vascular Carrier, Arteriovenous Shunt Loop or Arteriovenous Bundle, Has More Potential for Angiogenesis and Tissue Generation? Plast. Reconstr. Surg. 112:1636 2003 2) T.M.MacLeod, G.Williams, R.Sanders, et al. Prefabricated skin flaps in a rat model based on a dermal replacement matrix PermacolTM. Br. J. Plast. Surg. 53:775,2003
View Synopsis (.doc format, 95.0 kb)